Chemotherapy Inducted Peripheral Neuropathy (CIPN) – a side effect to therapeutic cancer treatment
You probably know someone who has been affected by cancer, whether it was a family member, friend or maybe even YOU. Regardless of the person, a cancer diagnosis and treatment can be overwhelming to endure and hard on family and friends. Patients are often affected by chemotherapy or radiation side effects that can be severe. Chemotherapy-Induced Peripheral Neuropathy (CIPN) is the most common side effect for someone undergoing chemotherapy treatment for breast cancer. The type of chemotherapeutic agents does influence the risk of developing neuropathies with reported CIPN rates as high as 85%. Many patients feel the side effects of CIPN are worse than the cancer treatments and would rather discontinue their treatments because of the severe neuropathy symptoms. In some cases, CIPN can develop shortly after finishing chemotherapy, known as “coasting.” In fact, 30 – 40% of patients will still have CIPN symptoms a year or more after finishing chemotherapy.
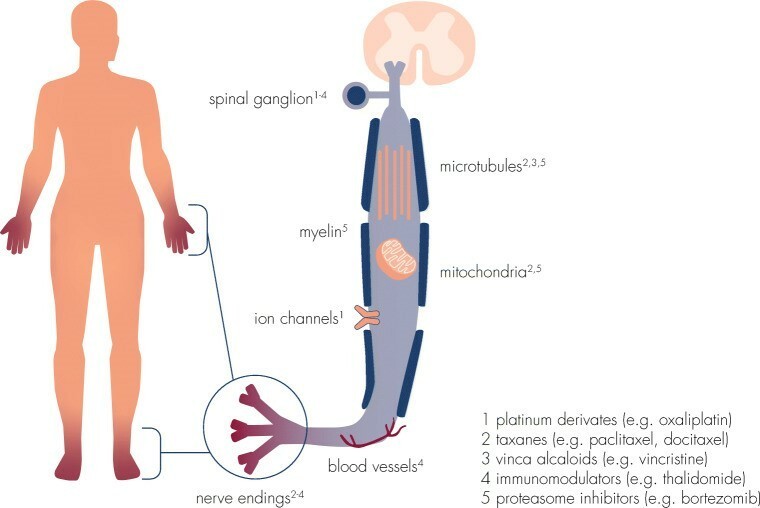
CIPN has no clear prevention options because neurotoxic antitumor agents cause damage to peripheral nerves (sensory, motor, and autonomic) which begin to occur during therapy and can remain in the body. Certain chemotherapeutic agents, (i.e., Cisplatin) can remain in the body for a long time producing peripheral nerve damage that develops into CIPN often in the hands and feet. Chemotherapeutic drugs exert neurotoxic effects on the myelin sheets (myelinopathy), on sensory cells bodies in the dorsal root ganglion (neuropathy), and on axonal components (axonopathy), including ion channels, microtubules, mitochondria, and associated capillaries.(Fig 1.) ¹
Fig 1. Neurotoxicity of different chemotherapeutic agents is mediated by interference with a variety of cellular structures and components of the peripheral nervous system.
CIPN represents a major concern in cancer therapy and a major challenge for oncology treatments. In the case of breast cancer, there are new chemotherapies that limit toxicity and improve efficacy. Analyzing a patient’s genetics phenotyping of the tumor provides a better understanding of targeted personalized treatment regimens. Other health risk factors associated with an increase in developing CIPN are older aged patients, other co-morbid health conditions, obesity, current prescribed medications, i.e., cardiovascular beta blockers, etc.
Due to the increase in cancer diagnosis with chemotherapy/radiation treatments to help improve cancer patient’s survival rates, CIPN is occurring even more frequently and is reported by medical oncologists as “almost a given condition” to patients undergoing chemotherapy. An innovative and advanced treatment to manage CIPN symptoms while patients undergo life-saving chemotherapy is available. Raja S. Mehdi, MD, Board-Certified Medical Oncologist and Founder of HOPE Cancer Care of Nevada helps his patients find relief from the excruciating pain of CIPN so they can finish their chemotherapy regimen and reduce their use of neuropathy pain medications. He refers his patients to Neuropathy and Pain Centers of Las Vegas, a pain management clinic that uses the RST-SANEXAS neoGEN® – Series Electric cell-Signaling Technology (EcST) to activate the recovery process.
Here is what Dr. Mehdi had to say.
“The release of new therapies for breast cancer means more patients living longer with better quality of life. Targeted therapies allow us to limit toxicity and improve efficacy. Analyzing the genetics of each patient’s tumor allows us to personalize each plan of care.”
“While the future for breast cancer treatment is promising, patients often experience chemotherapyinduced peripheral neuropathy (CIPN). Symptoms can be severe to the extent that chemotherapy patients would rather discontinue treatment. Such was the case for a recent patient of ours who was able to successfully continue their chemotherapy regimen while also being treated for CIPN with the use of the RST-SANEXAS neoGEN® Electric cell-Signaling Treatment (EcST) device at Neuropathy and Pain Centers of Las Vegas.”
Raja S. Mehdi, MD
Denise Miller-Tso, Nurse Practitioner and Owner of Atlanta Neuropathy Clinic offers her testimonial.
“I have found the device to be incredibly helpful for a myriad of chronic pain etiologies. One such etiology is pain associated with chemotherapy-induced peripheral neuropathy (CIPN). I find CIPN to be especially devastating because these patients have already endured so much. They had to process their mortality, then go through the daunting task of chemotherapy, and possibly radiation. The physical and mental toll on these patients is taxing, and just when they are grateful to complete their treatment, they’re left with a new problem: PAIN. The pain and numbness of CIPN diminishes the quality of life these patients just fought so hard for. They’re often given limited treatment options that fail to address the cause of their pain and further diminish their quality of life.”
“The CIPN patients at Atlanta Neuropathy Clinic have experienced incredible results from treatment with RST-SANEXAS. Patients report feeling like themselves again, “alive, not just surviving.” They report marked improvements in pain, numbness, burning, and cramping. Their mobility improves. They have hope again. Several of my CIPN patients have been able to wean off their gabapentin or Lyrica. RST-SANEXAS neoGEN® device and treatment protocols are truly life changing for CIPN patients.”
Denise Miller-Tso, Nurse Practitioner
Managing Chemotherapy Induced Peripheral Neuropathy
On a positive note, patients suffering with CIPN have RST-SANEXAS neoGEN® Electric cell-Signaling Treatments (EcST) that help relieve pain and other symptoms associated with CIPN but also with all other forms of neuropathy. The neoGEN® system produces complex electric signal energy waves to imitate, facilitate, exhaust, and interrupt the axonal nerve action potential. This FDA cleared device with its patented ultra-high digital frequency generator (UHdfg) delivers time-varying, targeted, and combined amplitude modulated (AM) and frequency modulated (FM) electric energy waves with associated harmonic resonance frequencies. These therapeutic pulsed energy waves penetrate deeper into the surrounding tissue and direct to the nerve cells. Because nerve cells are 60% more conductive than any other tissue or cells, their recovery processes begin in a comfortable, safe, non-invasive, non-pharmaceutical, and effective way when patients are being treated with the neoGEN® device. The typical treatment time is 10-30 minutes per session 2-3 times per week. The device’s 20 different programs provide various electric signal impulses creating physiological and therapeutic effects to relieve pain, increase circulation, and improve muscular rehabilitation.
Lymphedema is another problem that may occur after cancer surgery or chemotherapy/radiation treatment. Removal of the lymph glands causes disruption of the flow of lymph fluid, causing swelling. Lymphedema can happen any time after the lymph glands are removed and continue for years after cancer treatment ends. The pain and swelling associated with lymphedema can be managed with the RST-SANEXAS neoGEN® Electric cellSignaling treatment (EcST) device. The device increases local circulation to help manage the swelling in the affected area and control the pain.
A cancer diagnosis and treatment can be very frightening. Besides the emotional and physiological effects cancer has on a patient, the long-term severe side-effects of CIPN, lymphedema, or other painful conditions can be devastating. When everything looks dark, there is light at the end of the tunnel and HOPE is shining bright. For more information regarding the RST-SANEXAS neoGEN® device and advanced Electric cell-Signaling Technology (EcST) visit www.rstsanexas.com/science or call 702-315-2999 or 866-SANEXAS (866-726-3927).
Reference: 1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8236465/